
Our Science
Our Science
Ionis is a leader in developing transformational RNA-targeted therapeutics for hard-to-treat neurologic diseases.1,2
For decades, Ionis has worked to advance RNA-targeted technology to create therapeutics for rare and prevalent neurologic diseases.3-6
Ionis’ investigational therapeutics are designed to interact precisely with target RNA, resulting in investigational RNA-targeted therapeutics that may target the underlying cause of neurologic disease without altering patients’ genes.7,8
Ionis Is Continually Refining and Optimizing Its RNA-Targeted Therapeutic Platform to Positively Impact the Lives of Patients With Neurologic Diseases, Creating New Standards of Care3,8-10
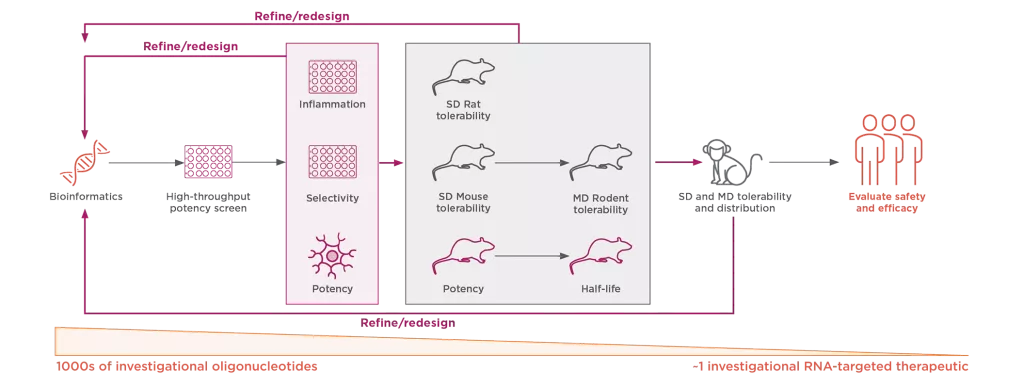
MD, multiple dose; SD, single dose.
Ionis has used knowledge from the genomics revolution to develop FDA-approved therapeutics for the treatment of spinal muscular atrophy, amyotrophic lateral sclerosis, and polyneuropathy in patients with hereditary transthyretin amyloidosis.5,11-16
References
- Ionis Pharmaceuticals. RNA-targeted medicines. Accessed March 4, 2025. https://ionis.com/science-and-innovation/
- Crooke ST. RNA-directed therapeutics at Ionis. Nature. 2019;574(7778):1-3.
- Ionis Pharmaceuticals. The creation of RNA targeting technology. December 2019. Accessed July 15, 2025. https://ir.ionispharma.com/static-files/1cada39c-0fa4-4e90-afdf-e85fa258e03b/
- Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20(6):427-453.
- Hua Y, Vickers TA, Baker BF, et al. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5(4):e73.
- Bennett CF, Krainer AR, Cleveland DW. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci. 2019;42:385-406.
- Quemener AM, Bachelot L, Forestier A, Donnou-Fournet E, Gilot D, Galibert MD. The powerful world of antisense oligonucleotides: from bench to bedside. Wiley Interdiscip Rev RNA. 2020;11(5):e1594.
- Crooke ST, Liang XH, Baker BF, Crooke RM. Antisense technology: a review. J Biol Chem. 2021;296:100416.
- Partridge W, Xia S, Kwoh TJ, Bhanot S, Geary RS, Baker BF. Improvements in the tolerability profile of 2'-O-methoxyethyl chimeric antisense oligonucleotides in parallel with advances in design, screening, and other methods. Nucleic Acid Ther. 2021;31(6):417-426.
- Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics [published correction appears in Cell Metab. 2019 Feb 5;29(2):501. doi: 10.1016/j.cmet.2019.01.001]. Cell Metab. 2018;27(4):714-739.
- AstraZeneca. Press release. December 7, 2021. Accessed July 15, 2025. https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-ionis-to-collaborate-on-eplontersen.html#/
- FDA approves first drug for spinal muscular atrophy. US Food and Drug Administration. December 23, 2016. Accessed July 15, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy/
- Hua Y, Vickers TA, Okunola HL, Bennett CF, Krainer AR. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet. 2008;82(4):834-848.
- Qiu J, Wu L, Qu R, et al. History of development of the life-saving drug "Nusinersen" in spinal muscular atrophy. Front Cell Neurosci. 2022;16:942976.
- Miller TM, Cudkowicz ME, Genge A, et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099-1110.
- Wainua. Package insert. AstraZeneca. Updated September 2024. Accessed July 15, 2025. https://www.azpicentral.com/pi.html?product=wainua
