RNA-Targeted Regulation
of Proteins
RNA-Targeted Regulation
of Proteins
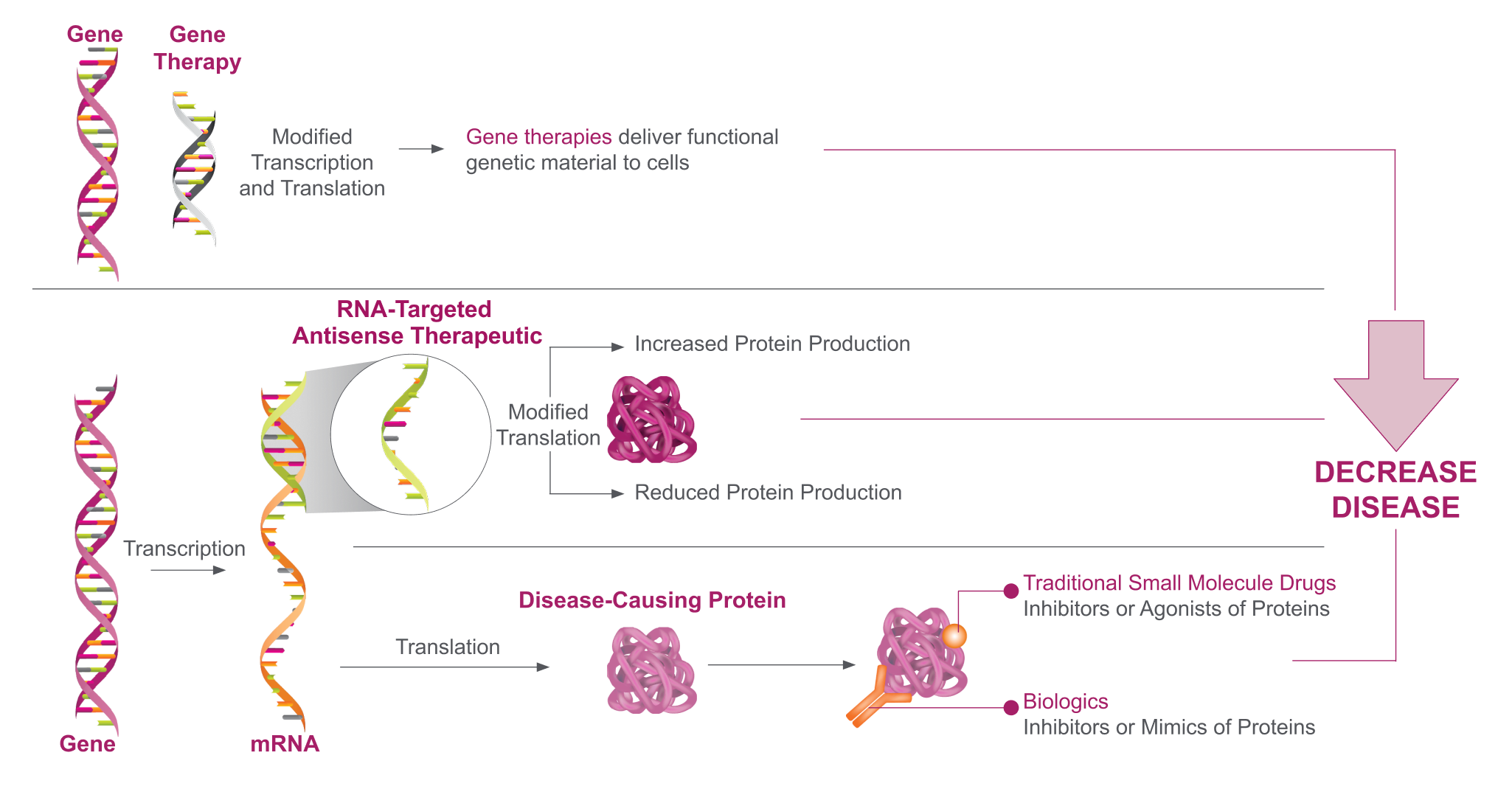
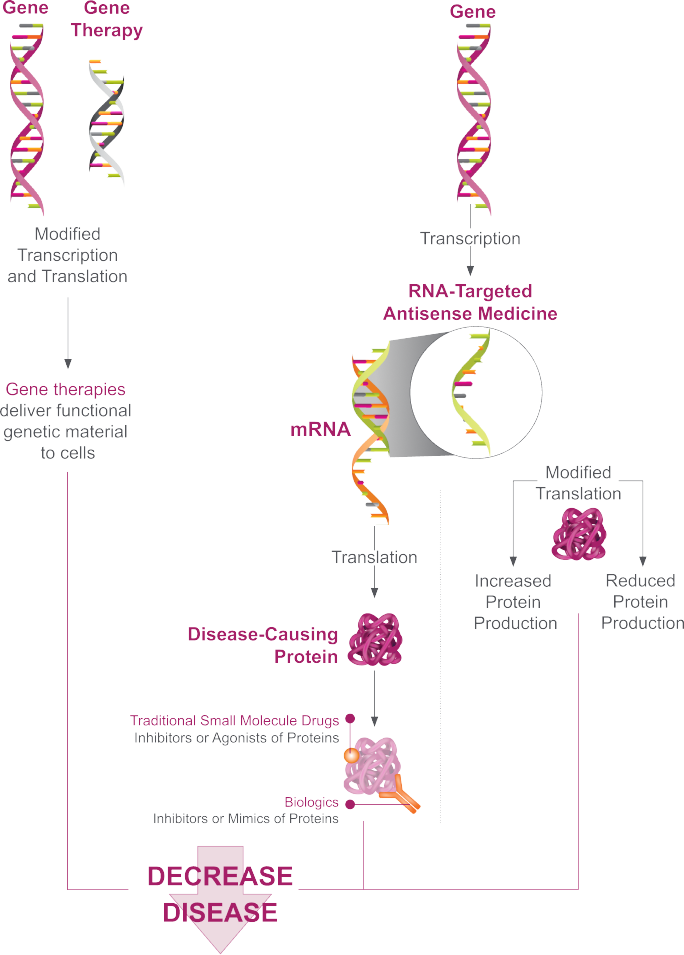
Once a causative gene is identified, researchers can use this knowledge to develop RNA-targeted therapeutics that target and modulate the associated mRNA, thereby altering protein production.1-8
RNA-targeted therapeutics are designed to interact precisely with RNA through Watson-Crick base pairing, allowing them to target a single disease-associated gene throughout different cell types within the central nervous system.8,9
Neurologic diseases that may not be amenable to treatment with small molecules or biologics may be treated with RNA-targeted therapeutics.1,2,4-8 The effects of RNA-targeted therapeutics on gene expression are rapid and reversible, and they do not modify a patient’s genome.2,10,11
RNA-Targeted Therapeutics Alter Gene Expression Upstream of Protein Production Without Altering a Patient’s Genome4,5,7,8,12,13
Antisense Oligonucleotides Are a Class of RNA-Targeted Therapeutics
Antisense Oligonucleotides Are a Class of RNA-Targeted Therapeutics
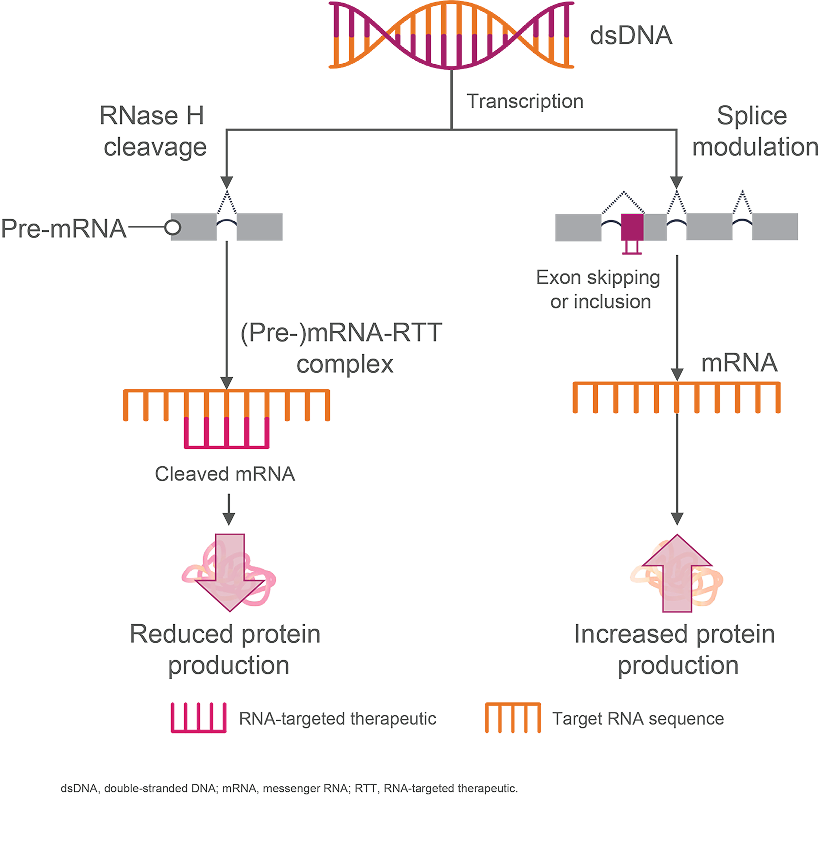
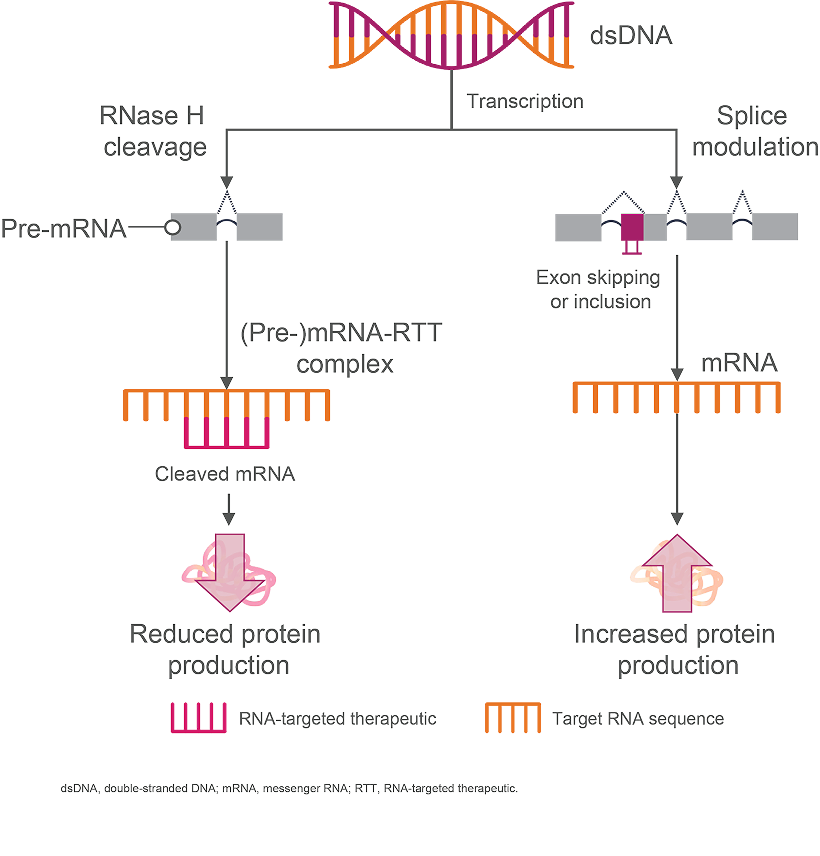
Antisense oligonucleotides (ASOs) are single-strand oligonucleotides that bind directly to their mRNA target to upregulate or downregulate protein production by either2,11,14:
- Preventing protein translation through RNase H-mediated degradation of the heteroduplex (ie, RNA-targeted therapeutic–[pre-]mRNA duplex)
- Regulating gene expression via heteroduplex-induced alternative splicing, which includes exon skipping or inclusion
How Antisense Oligonucleotides Are Proposed to Work11,15
Harnessing the power of RNA, Ionis has developed FDA-approved RNA-targeted therapeutics for the treatment of spinal muscular atrophy (SMA), superoxide dismutase 1-amyotrophic lateral sclerosis, a genetic form of the disease, and polyneuropathy in patients with hereditary transthyretin amyloidosis.16-22
Case Study: Spinal Muscular Atrophy (SMA)
Case Study: Spinal Muscular Atrophy (SMA)
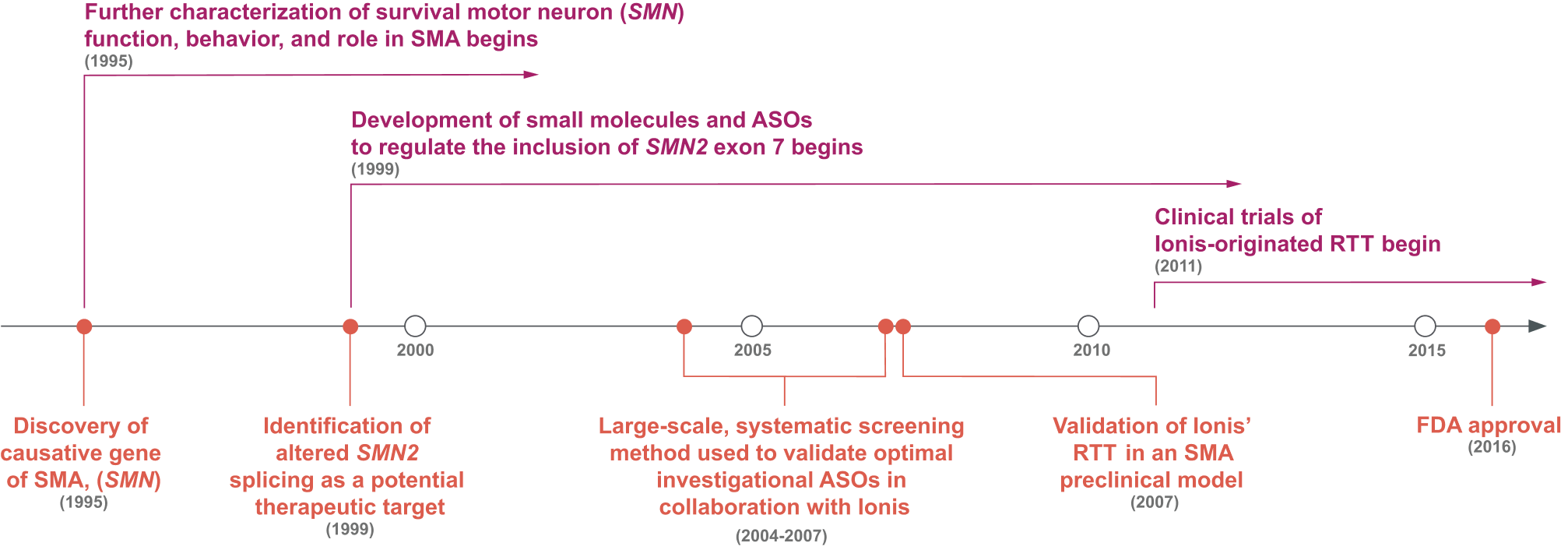
Prior to the 1990s, the fundamental cause of SMA was unknown.16,23 Research throughout the 1990s identified the cause of SMA—the deletion of survival of motor neuron 1 gene (SMN1)—and a potential therapeutic target: alternative splicing to include exon 7 of SMN2.16,18
ASOs Were Screened for the Optimal Target Along Exon 7 of SMN2, Marking a Shift in Drug Discovery.5,16-18,24,25
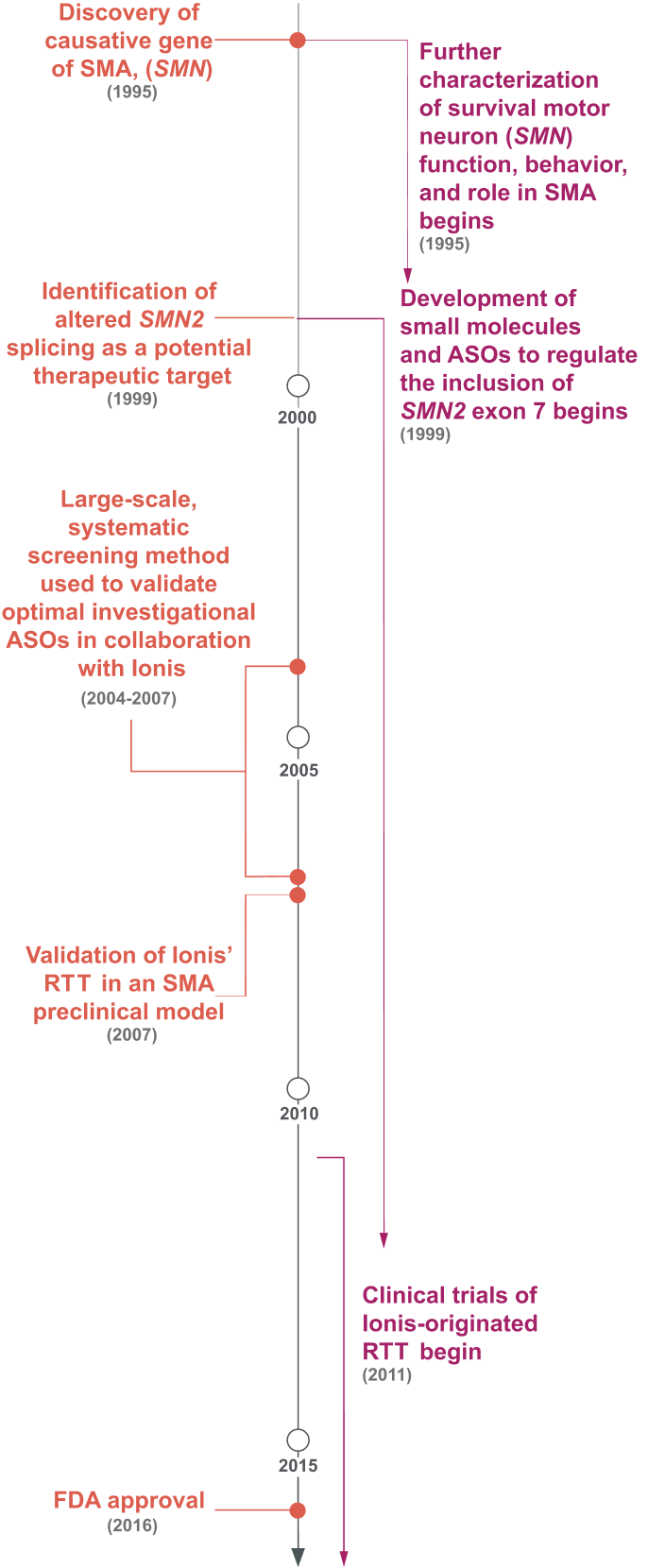
ASO, antisense oligonucleotide; FDA, US Food and Drug Administration; RTT, RNA-targeted therapeutic; SMA, spinal muscular atrophy.
Learn more about how Ionis' RNA-targeted therapeutics have been shown to work in animals.
References
- Bennett CF, Krainer AR, Cleveland DW. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci. 2019;42:385-406.
- Crooke ST, Liang XH, Baker BF, Crooke RM. Antisense technology: a review. J Biol Chem. 2021;296:100416.
- Poplawski SG, Garbett KA, McMahan RL, et al. An antisense oligonucleotide leads to suppressed transcription of Hdac2 and long-term memory enhancement. Mol Ther Nucleic Acids. 2020;19:1399-1412.
- Coleman N, Rodon J. Taking aim at the undruggable. Am Soc Clin Oncol Educ Book. 2021;41:1-8.
- Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20(6):427-453.
- Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673-694.
- Yu AM, Jian C, Yu AH, Tu MJ. RNA therapy: are we using the right molecules? Pharmacol Ther. 2019;196:91-104.
- Chery J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 2016;4(7):35-50.
- Jafar-Nejad P, Powers B, Soriano A, et al. The atlas of RNase H antisense oligonucleotide distribution and activity in the CNS of rodents and non-human primates following central administration. Nucleic Acids Res. 2021;49(2):657-673.
- Kher G, Trehan S, Misra A. Antisense oligonucleotides and RNA interference. In: Misra A, ed. Challenges in Delivery of Therapeutic Genomics and Proteomics. Elsevier; 2011:325-386.
- Bennett CF, Kordasiewicz HM, Cleveland DW. Antisense drugs make sense for neurological diseases. Annu Rev Pharmacol Toxicol. 2021;61:831-852.
- Martier R, Konstantinova P. Gene therapy for neurodegenerative diseases: slowing down the ticking clock. Front Neurosci. 2020;14:580179.
- Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2020;383(2):109-119.
- Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics [published correction appears in Cell Metab. 2019;29(2):501. doi: 10.1016/j.cmet.2019.01.001]. Cell Metab. 2018;27(4):714-739.
- Bajan S, Hutvagner G. RNA-based therapeutics: from antisense oligonucleotides to miRNAs. Cells. 2020;9(1):137.
- Qiu J, Wu L, Qu R, et al. History of development of the life-saving drug “Nusinersen” in spinal muscular atrophy. Front Cell Neurosci. 2022;16:942976.
- Hua Y, Vickers TA, Baker BF, et al. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5(4):e73.
- Hua Y, Vickers TA, Okunola HL, Bennett CF, Krainer AR. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet. 2008;82(4):834-848.
- FDA approves first drug for spinal muscular atrophy. US Food and Drug Administration. December 23, 2016. Accessed July 15, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy/
- AstraZeneca. Press release. December 7, 2021. Accessed July 15, 2025. https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-ionis-to-collaborate-on-eplontersen.html#/
- Wainua. Package insert. AstraZeneca. Updated September 2024. Accessed July 15, 2025. https://www.azpicentral.com/pi.html?product=wainua
- Miller TM, Cudkowicz ME, Genge A, et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099-1110.
- Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. 2015;33(4):831-846.
- An open-label, escalating dose study to assess the safety, tolerability and dose-range finding of a single intrathecal dose of ISIS 396443 in patients with spinal muscular atrophy. ClinicalTrials.gov identifier: NCT01494701. Updated February 18, 2021. Accessed July 15, 2025. https://www.clinicaltrials.gov/study/NCT01494701/
- Partridge W, Xia S, Kwoh TJ, Bhanot S, Geary RS, Baker BF. Improvements in the tolerability profile of 2′-O-methoxyethyl chimeric antisense oligonucleotides in parallel with advances in design, screening, and other methods. Nucleic Acid Ther. 2021;31(6):417-426.